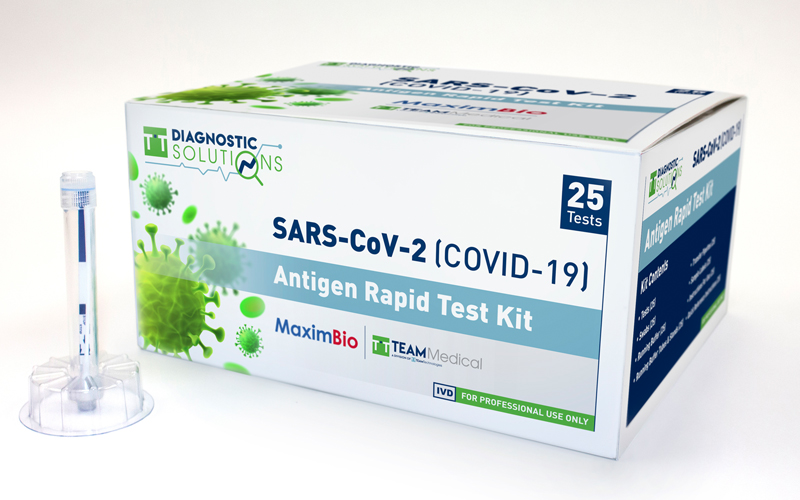
TEAM Technologies to Produce Rapid Antigen COVID-19 Tests
TEAM Technologies Leverages Medical Device Capabilities in Partnership with Maxim Biomedical to Produce Rapid Antigen COVID-19 (SARS-CoV-2) Tests that Deliver Results in 15 Minutes
Developed in conjunction with Maxim Biomedical Inc. and the National Institutes of Health (NIH), the Rapid Antigen Diagnostic Tests offer Fast and Easy-to-Read Results Ideal for Point-of-Care (POC) Use
Morristown, TN — September 8, 2020 — As part of its growing portfolio of medical device and healthcare solutions, TEAM Technologies will produce point-of-care (POC) tests to assist organizations seeking the benefits of rapid testing for COVID-19. TEAM Technologies is producing the in-vitro diagnostic (IVD) test with Maxim Biomedical, who developed the test as part of the NIH Rapid Acceleration of Diagnostics RADxSM initiative, a program launched last April to speed innovation in the development, commercialization, and implementation of testing technologies for COVID-19.
TEAM Technologies has facilities across the United States providing a variety of services ranging from product development, supply line expertise, and packaging and kitting of the SARS-CoV-2 diagnostic tests. “We are pleased to be working with the team at Maxim Biomedical as an instrumental partner in bringing this much needed testing solution to market,” commented Marshall White, President and CEO of TEAM Technologies. Collectively, TEAM and Maxim currently have a goal to produce 3 million tests by year’s end and to achieve a production rate of 15 million tests per month in early 2021.
The SARS-CoV-2 Rapid Antigen Diagnostic Test utilizes Lateral Flow Assay (LFA) technology with a streamlined workflow and a closed-tube format that eliminates the need for expensive equipment or a reader to perform. The assay achieves this by combining the specimen swab, reagents, and test strip in a compact, self-contained environment, allowing for incubation, reading and disposal in one tube. Results are available within 15 minutes and stable for more than two hours.
Complementing the IVD and POC experience of Maxim Biomedical, TEAM Technologies is employing its medical device manufacturing capabilities to rapidly scale production quantities of the SARS-CoV-2 rapid antigen test. “Many organizations, from schools to businesses to factories, can benefit from having the ability to perform testing that produces safe, quick and easy-to-interpret results,” White added. “We are proud to partner with our colleagues at Maxim Biomedical to make this test available.”
Mr. Jonathan Maa, COO of Maxim Biomedical, Inc. said, “Maxim Biomedical has always endeavored to provide high quality testing where it would fulfil unmet needs. The urgent demand for testing is an opportunity to apply our expertise in LFA technology. We developed a point-of-care rapid antigen test that is safe for community use and reduces risk for patients and front-line workers. We are glad we found a partner like TEAM Technologies to help us bring this important test to market.”
As part of an effort to boost COVID-19 testing capacity, this project has been funded in part by the NIH Rapid Acceleration of Diagnostics (RADx SM) initiative with federal funds from the National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health, Department of Health and Human Services, under Contract No. 75N92020C00020.
This SARS-CoV-2 Rapid Antigen Diagnostic Test has not been FDA cleared or approved.
About TEAM Technologies
Headquartered in Morristown, TN with 13 facilities throughout the United States, TEAM Technologies is a leading solutions provider supporting the foremost global healthcare products companies. With its ‘ONE TEAM, MANY TECHNOLOGIES’ philosophy, TEAM boasts an extensive lineup of manufacturing processes designed to service the oral care, infection prevention and control, advanced wound care, pharmaceutical dispensing, IVD diagnostics and other medical end markets. With an entrepreneurial mindset and a management team with deep industry experience, TEAM Technologies leverages seamless, turnkey processes and innovation to positively impact the success of TEAM’s customers. For more information, visit teamtech.com.
About Maxim Biomedical Inc.
Founded in 2005 as a branch of the Maxim family of businesses, Maxim Biomedical (www.maximbio.com) was created as a company dedicated to the development of high quality, in-vitro diagnostic (IVD) and Point-of-Care (POC) testing solutions that make positive contributions to public health and healthcare worldwide. The company, located in Rockville, Maryland places Maxim Biomedical in one of the world’s top scientific regions, just steps away from our nation’s capital. Maxim Biomedical’s 26,000 square foot facility houses over 13 individual laboratories. The company offers a line of FDA and CDC approved products, as well as raw materials, and customized CRO/CMO services.
Forward Looking Statements
This press release contains forward-looking statements within the meaning of the federal securities laws that involve material risks, assumptions and uncertainties. Many possible events or factors could affect our future financial results and performance, such that our actual results and performance may differ materially from those that may be described or implied in the forward-looking statements. As such, no forward-looking statement can be guaranteed. Differences in actual results and performance may arise as a result of a number of factors including, without limitation: the impact of the novel virus (COVID-19) global pandemic; an inability to agree on a definitive agreement or meet key deliverables and milestones under the NIH’s RADx-ATP contract.